Glycosylation modification is one of the most important and complex modifications in numerous protein post-translational modifications and is one of the key quality attributes for antibody evaluation. The realization of monoclonal antibody function is closely related to its glycosylation modification, which affects protein properties such as conformation, stability, solubility, pharmacokinetics, activity and immunogenicity. In this article, the author gives a brief overview of glycosylation and its stability/half-life, safety and biological activity of antibody drugs.
Glycosylation is one of the important post-translational modifications of proteins. According to the glycosylation modification site, glycosylation can be divided into N-position glycosylation and O-position glycosylation. The N-glycosylation is located in Asn-297, the N-acetylglucosamine in the oligosaccharide and the amide nitrogen-linked modified protein on the asparagine residue, starting from the endoplasmic reticulum in the Golgi apparatus; The N-acetylgalactose in the oligosaccharide is linked to the hydroxyl group on the serine or threonine residue to modify the protein, which is completed in the Golgi apparatus.
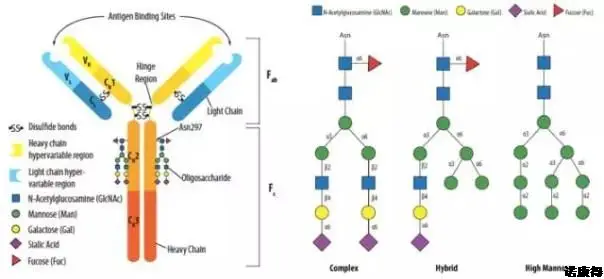
The N-glycosylation of immunoglobulins secreted by animal cells is the most common glycosylation modification and the most studied glycosylation modification. Taking IgG1 as an example, its important glycosylation modification site is located at the Fc end, and can be divided into complex, heterozygous and high mannose types according to the fine structure (length, branch and monosaccharide arrangement) of the terminal. ,As shown in Figure 1.
Figure 1 Location of N-glycosylation in the monoclonal antibody (left) and its three main types (right)
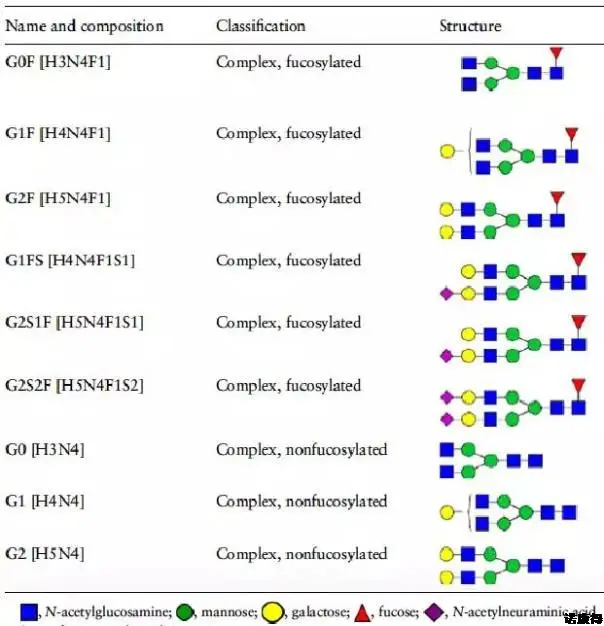
In Figure 1, it can be seen that the glycosylation of IgG1 is a complex type, the glycosylation is based on fucose (Fuc), and N-acetylglucosamine (N-GlcNAc) is divided into two equal lengths. Branches, which are branched with mannose (Man), galactose (Gal), and sialic acid (Sia), thereby constituting the N-position glycosylation of the Fc end of IgG1. According to the different monosaccharides in the two branches, the glycosylation modification type of IgG1 can be divided into different glycoforms in Table 1. It is the difference in glycoform that leads to the immunogenicity, biological activity, and drug power of the monoclonal antibody. The difference between learning and so on.
Table 1 Types of Fc-terminal glycosylation of IgG1
Next:没有了